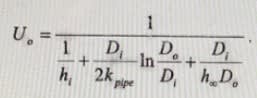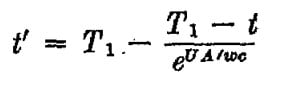Hii everyone .
i am designing an hydroponic system , and there is one calculation that really gives me a hard time , i will be very happy if someone can help me with this. I simplified the equation that i need to develop down below. thank you very much for any help.
Question
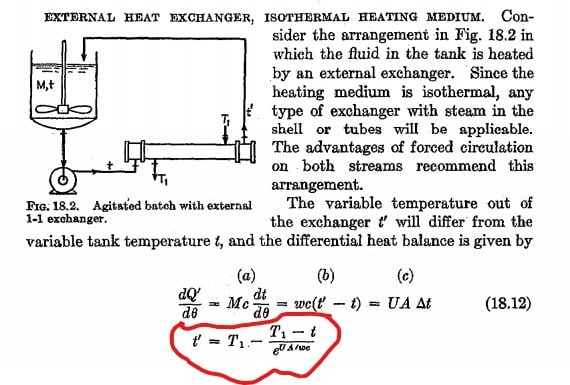
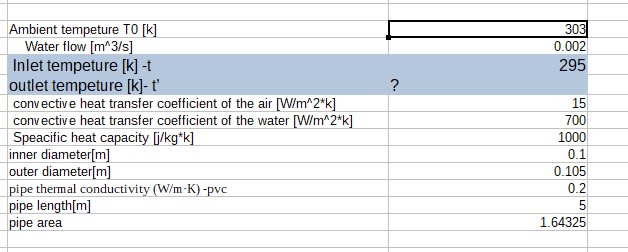
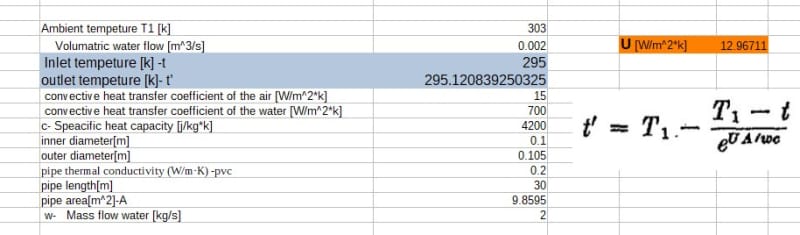
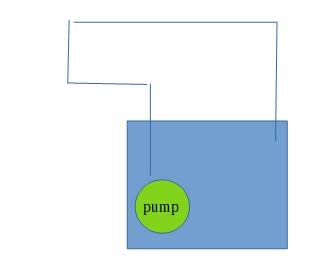
An isolated tank contains M amount of water. A pipe connected to the tank with a pump, delivering the water in a close circle back to the tank through the atmosphere. Assuming the water temperature distribution is equal in the tank at the beginning . Create an equation of the temperature in the tank relative to the time .
Given :
water flow through the pipe 3M/h (three times the water tank per hour)
Initial temperature at the tank T1
Atmosphere temperature T0
Length of the pipe L
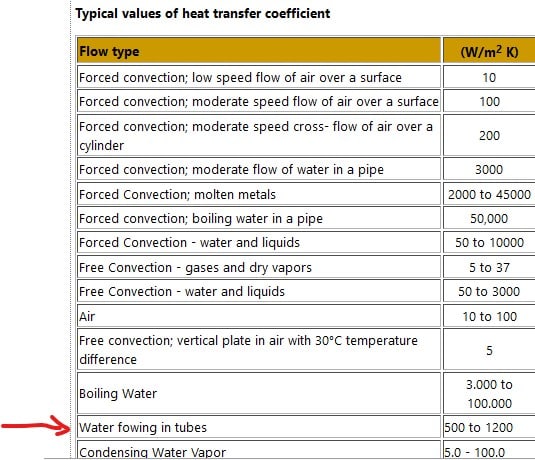
h0 - convective heat transfer coefficient of the air
hW -convective heat transfer coefficient of the water
k- pipe thermal conductivity (W/m·K)
d – pipe diameter
*Can use any other obvious data that is needed and can be reached easily online .
Please explain every step, equation and assumption that is used.
i am designing an hydroponic system , and there is one calculation that really gives me a hard time , i will be very happy if someone can help me with this. I simplified the equation that i need to develop down below. thank you very much for any help.
Question
An isolated tank contains M amount of water. A pipe connected to the tank with a pump, delivering the water in a close circle back to the tank through the atmosphere. Assuming the water temperature distribution is equal in the tank at the beginning . Create an equation of the temperature in the tank relative to the time .
Given :
water flow through the pipe 3M/h (three times the water tank per hour)
Initial temperature at the tank T1
Atmosphere temperature T0
Length of the pipe L
h0 - convective heat transfer coefficient of the air
hW -convective heat transfer coefficient of the water
k- pipe thermal conductivity (W/m·K)
d – pipe diameter
*Can use any other obvious data that is needed and can be reached easily online .
Please explain every step, equation and assumption that is used.