Hello Everyone,
Would someone here help me with this question:
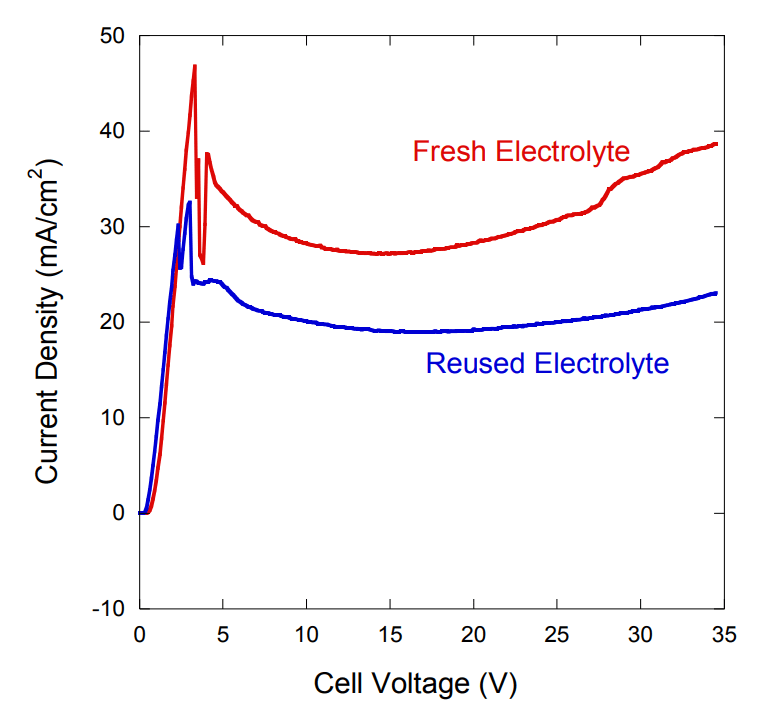
What happens to the Electrical Conductivity of the electrolyte when the electropolishing solution ages?
Will it increase or decrease?
I am considering a mixture of Sulphuric acid, Phosphoric acid and water.
Thanks.
Would someone here help me with this question:
What happens to the Electrical Conductivity of the electrolyte when the electropolishing solution ages?
Will it increase or decrease?
I am considering a mixture of Sulphuric acid, Phosphoric acid and water.
Thanks.