Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
-
Congratulations JAE on being selected by the Eng-Tips community for having the most helpful posts in the forums last week. Way to Go!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
pressure inside the CO2 cylinder
- Thread starter sal10
- Start date
- Status
- Not open for further replies.
- Thread starter
- #2
The pressure in a spun steel cylinder, filled with CO2 will be affected by ambient temperature, but it won't be more than 800psi.
Regards,
DM
"Real world Knowledge isn't dropped from a parachute in the sky but rather acquired in tiny increments from a variety of sources including panic and curiosity."
Regards,
DM
"Real world Knowledge isn't dropped from a parachute in the sky but rather acquired in tiny increments from a variety of sources including panic and curiosity."
Rather late reply but it wont hurt anybody.
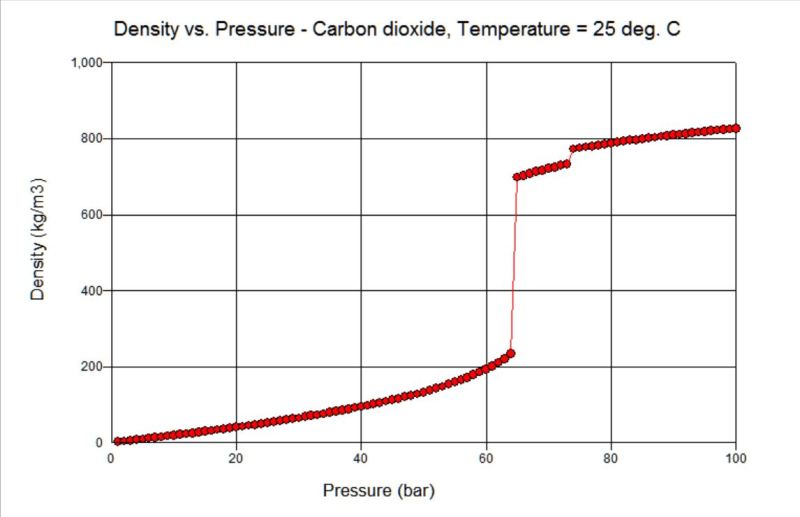
At least from a theoretical point of view, the relation of pressure and density of CO2 at 25C (77F) is shown below.
or
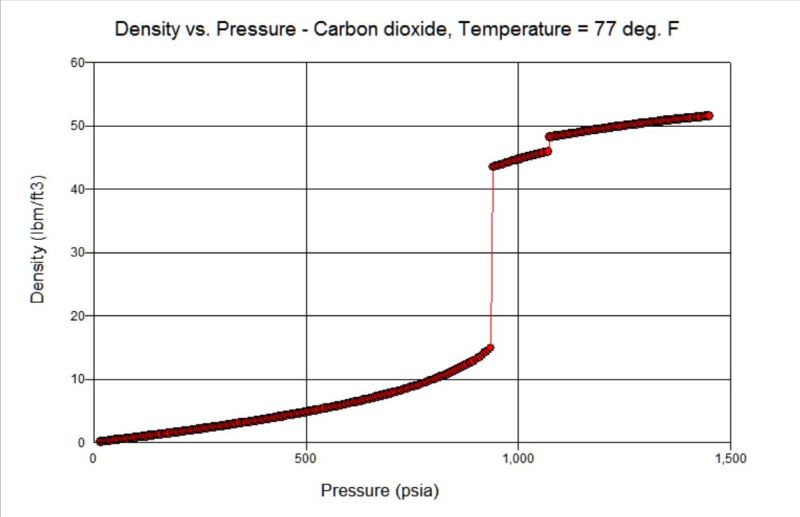
It turns out as shown above that with a 500gr/m3 density (31.21 lb/ft3), CO2 is on a saturated state between liquid and vapor at 64.45 bar (935 psi).
At least from a theoretical point of view, the relation of pressure and density of CO2 at 25C (77F) is shown below.

or

It turns out as shown above that with a 500gr/m3 density (31.21 lb/ft3), CO2 is on a saturated state between liquid and vapor at 64.45 bar (935 psi).
LittleInch
Petroleum
CO2 is weird stuff.
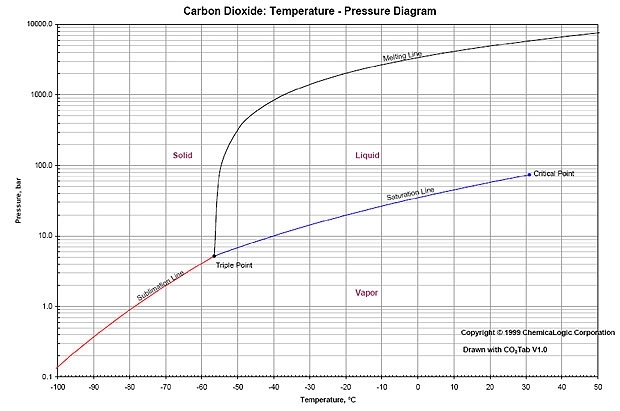
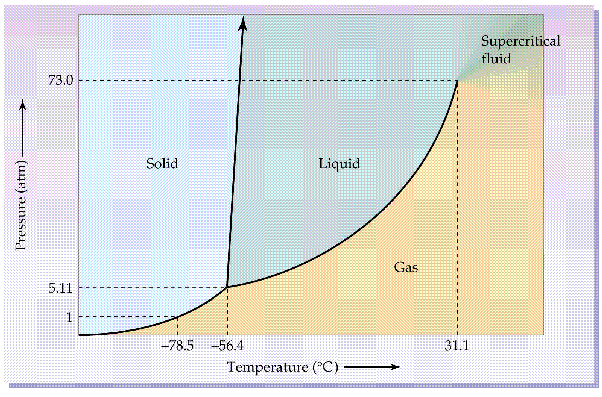
Below the magic critical point of 31.1C, the pressure temperature line should follow the critical path line.
As the temperature changes, the pressure changes along that line when you have liquid and gas. So as you release gas the pressure and temperature falls until heat from outside heats up the liquid to then equalize the pressure.
So quantity is irrelevant so long as there is some liquid in a sealed container.
So for 25C the pressure is around 65 bar.
Beyond 31.1C it all gets a bit weird. (super critical) so can get higher pressures.
Remember - More details = better answers
Also: If you get a response it's polite to respond to it.
Below the magic critical point of 31.1C, the pressure temperature line should follow the critical path line.
As the temperature changes, the pressure changes along that line when you have liquid and gas. So as you release gas the pressure and temperature falls until heat from outside heats up the liquid to then equalize the pressure.
So quantity is irrelevant so long as there is some liquid in a sealed container.
So for 25C the pressure is around 65 bar.
Beyond 31.1C it all gets a bit weird. (super critical) so can get higher pressures.


Remember - More details = better answers
Also: If you get a response it's polite to respond to it.
- Status
- Not open for further replies.
Similar threads
- Replies
- 0
- Views
- 301
- Replies
- 1
- Views
- 1K
- Replies
- 0
- Views
- 359
- Question
- Replies
- 2
- Views
- 437
- Locked
- Question
- Replies
- 6
- Views
- 2K
