Prometheus21
Mechanical
Hello everyone,
As my knowledge on this topic is lacking, and I'm heavily in need of a refresher course; some input from you as well will likely be very helpful.
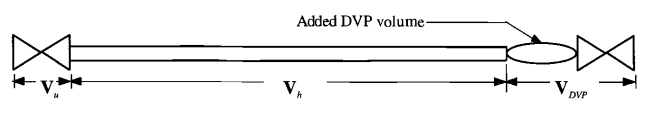
For the sake of discussion I am looking at simple system; a DN6 flexible polymer-lined flex-hose with a distance/volume piece (heat-sink) at the end. The hose is to be pressurized with 360 bar oxygen (99,5%) at 60°C at a timespan of 20 ms or less. In other words we are looking at an adiabatic compression; or gas hammer effect.
Now designing this heat-sink is rather important to contain the initial slug of gas; and the first compression event is the most severe one; as subsequent compressions does not achieve nearly as great a temperature as the slug that is initially present. A key feature in this configuration is that there is very little mixing of the two gas slugs (the initial slug and the incoming one). Mathematically this compression is usually treated as if a piston was performing the compression (located at the interface of the to gas slugs).
Now there are guidelines in designing a heat-sink with regards to the volume of the initial compression; the consensus being that a short, large-diameter distance piece can be functionally equivalent to a long small-diameter distance piece.
If the slug of gas reaches a high enough temperature then this can lead to ignition of contaminants or the hose itself; meaning the length of this hot slug must be contained.
How would one estimate (with a high level of accuracy):
1. The minimum required length for a given internal diameter D of the heat sink?
2. The volume of the hot gas slug?
Any input on pointers or references that might help me out here? I could always run CFDs but I need to get a firmer grip on the theory behind it.
Thank you.
As my knowledge on this topic is lacking, and I'm heavily in need of a refresher course; some input from you as well will likely be very helpful.
For the sake of discussion I am looking at simple system; a DN6 flexible polymer-lined flex-hose with a distance/volume piece (heat-sink) at the end. The hose is to be pressurized with 360 bar oxygen (99,5%) at 60°C at a timespan of 20 ms or less. In other words we are looking at an adiabatic compression; or gas hammer effect.
Now designing this heat-sink is rather important to contain the initial slug of gas; and the first compression event is the most severe one; as subsequent compressions does not achieve nearly as great a temperature as the slug that is initially present. A key feature in this configuration is that there is very little mixing of the two gas slugs (the initial slug and the incoming one). Mathematically this compression is usually treated as if a piston was performing the compression (located at the interface of the to gas slugs).
Now there are guidelines in designing a heat-sink with regards to the volume of the initial compression; the consensus being that a short, large-diameter distance piece can be functionally equivalent to a long small-diameter distance piece.
If the slug of gas reaches a high enough temperature then this can lead to ignition of contaminants or the hose itself; meaning the length of this hot slug must be contained.
How would one estimate (with a high level of accuracy):
1. The minimum required length for a given internal diameter D of the heat sink?
2. The volume of the hot gas slug?
Any input on pointers or references that might help me out here? I could always run CFDs but I need to get a firmer grip on the theory behind it.
Thank you.