As you can see, both equations use the ideal gas low with the universal gas constant in J/kg.k
Both equations are used in published articles as follows:
"Application of dynamic programming to the optimal management of a hybrid power plant with wind turbines, photovoltaic panels and compressed air energy storage" and "Design and thermodynamic analysis of a multi-level underwater compressed air energy storage system"
Should the air specific heat ratio "K" be included in the ideal gas law or not ?

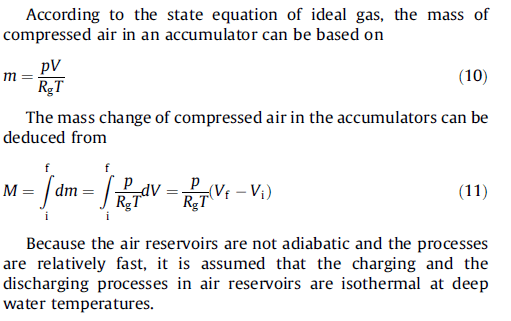
Both equations are used in published articles as follows:
"Application of dynamic programming to the optimal management of a hybrid power plant with wind turbines, photovoltaic panels and compressed air energy storage" and "Design and thermodynamic analysis of a multi-level underwater compressed air energy storage system"
Should the air specific heat ratio "K" be included in the ideal gas law or not ?


