Hi everyone.
Which methods are correct to determine salt content in PTB (pounds per thousand barrel)?
I have done a test to determine the value of Chloride for Crude oil entering Desalter using Mercury Nitrate titration method. The value of Chloride is 10 ppm.
My laboratory method to determine salt content (ptb) is 10 ppm Chloride x 1.65 divide by 2.853 which yield 5.78 ptb.
However, when i check Oilfield Processing of Petroleum - Volume 2 - Manning & Thompson book, the equation use is very different. I need to put BS&W value. The equation is
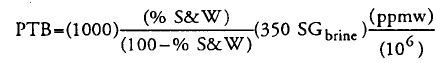
Assuming BS&W in = 0.5 vol%, SG = 1.02 and Chloride ppm = 10 ppm, therefore the value would be 0.018 ptb.
Which one of these equations are correct to determine salt content in PTB? I your guidance.
Which methods are correct to determine salt content in PTB (pounds per thousand barrel)?
I have done a test to determine the value of Chloride for Crude oil entering Desalter using Mercury Nitrate titration method. The value of Chloride is 10 ppm.
My laboratory method to determine salt content (ptb) is 10 ppm Chloride x 1.65 divide by 2.853 which yield 5.78 ptb.
However, when i check Oilfield Processing of Petroleum - Volume 2 - Manning & Thompson book, the equation use is very different. I need to put BS&W value. The equation is
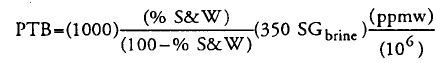
Assuming BS&W in = 0.5 vol%, SG = 1.02 and Chloride ppm = 10 ppm, therefore the value would be 0.018 ptb.
Which one of these equations are correct to determine salt content in PTB? I your guidance.
