mechengineer
Mechanical
Hi Experts,
Condition: Pressure vessel and Sea water service.
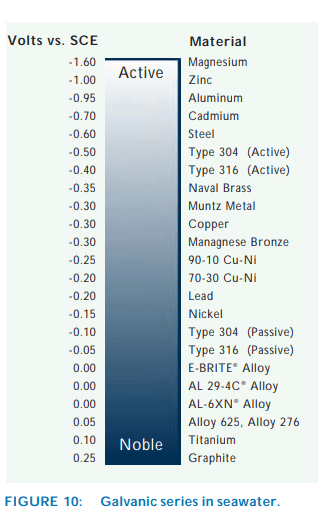
The flange material: SB-283 C70620.
The bolt materials: SS316 or duplex/supper duplex or Monel.
Question: will it be gavonized corrosion between SB-283 C70620 and SS316 or duplex/supper duplex or Monel.
Thanks,
Condition: Pressure vessel and Sea water service.
The flange material: SB-283 C70620.
The bolt materials: SS316 or duplex/supper duplex or Monel.
Question: will it be gavonized corrosion between SB-283 C70620 and SS316 or duplex/supper duplex or Monel.
Thanks,