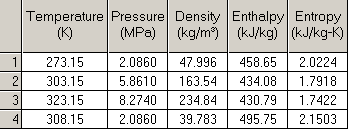
CO2 enters the first pump at 300psi 0C, exits at 850psi 30C, enters the second pump, exits at 1200psi 50C. What temperature would it exit the second pump at if the initial temp was 35C?
I've searched the forums, textbooks and after several attempts of calculating I don't think I have the right approach. Any suggestions would be very appreciated.
I've searched the forums, textbooks and after several attempts of calculating I don't think I have the right approach. Any suggestions would be very appreciated.