processendbd
Chemical
I know selectivity is = No of mole of desired product formed/ No of mole of reactant took part in the reaction (reactant Basis)
Ethylene Oxide example
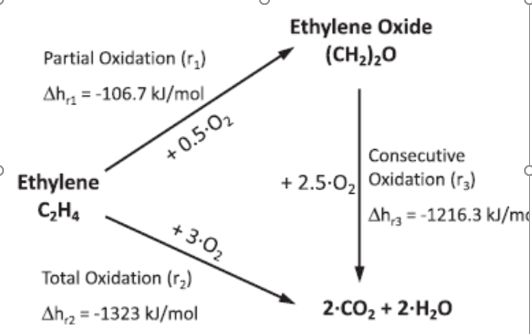
Considering only the first two reactions (No further oxidation of EO)
Assume 153 Kmol/hr of EO forms
So if I have 90% selectivity that means I will have 34 Kmol/hr of CO2. (As every 1 mole ethylene produces 2 mol of CO2)
Another thing selectivity is for the overall process so with recycle right?
Much appreciated.
Ethylene Oxide example

Considering only the first two reactions (No further oxidation of EO)
Assume 153 Kmol/hr of EO forms
So if I have 90% selectivity that means I will have 34 Kmol/hr of CO2. (As every 1 mole ethylene produces 2 mol of CO2)
Another thing selectivity is for the overall process so with recycle right?
Much appreciated.
