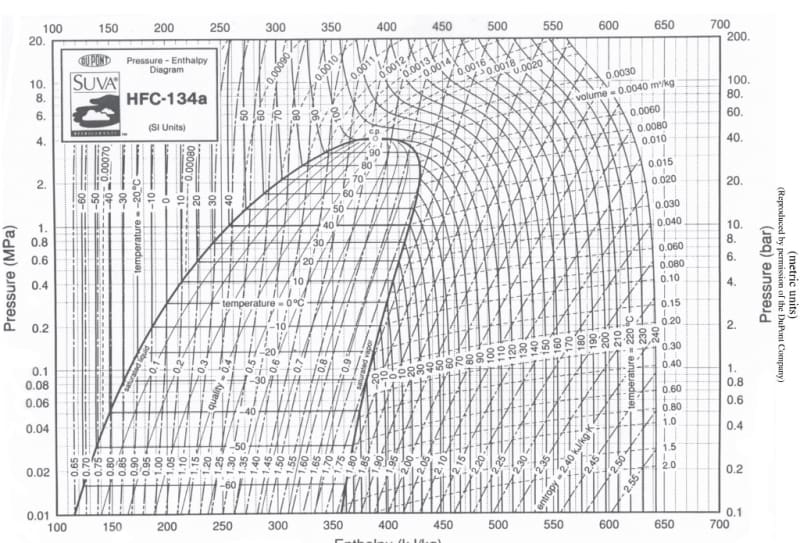
Hi, I've designed a stainless steel cylinder cell to measure the electrical conductivity of R134a in liquid phase (under pressure). I'm a mechanical engineer and not a chemical engineer so i was wondering if you guys can kindly help me out with this. Initially, the procedure we were hoping to implement was to fill from a valve at the bottom (see picture attached) and wait before opening the top valves slightly to see whether liquid is coming out to ensure the refrigerant is in liquid phase before carrying out the experiment (measuring current from electrode inside the cell). The problem is, when opening the top valve, the flash evaporation caused the whole cell to cool significantly (roughly 50C temperature drop, calculated using Clausius–Clapeyron equation) which affected our readings because we want to measure at 25C. The saturation pressure of 134a is 665kPa, it gets released to atmospheric conditions.
I've been thinking about adding a pressure sensor and thermocouple between the top valves (see pic) to use the pressure reading as an indicator of liquid phase, instead of opening it from the top. Then, I would release the liquid from the same bottom valve I filled cell from into an empty cylinder with a relatively long pipe in between, to ensure the cooling effect does not reach the cell. Is there anything you think i can do further to reduce the throttling effect in my system? There must be a way of removing the liquid with a gradual decrease in pressure that stops throttling from occurring when opening a valve right?
I was thinking of covering the valves and pipes at the bottom and top of the cell with heat tapes (set to 25C) as well to limit the throttling. The problem with this though is that a temperature gradient could develop maybe causing bubbles to rise to the top of the cell which would affect our reading (we need uniform liquid conditions). Our cell is within a stainless steel cylinder enclosure to hold it together while being pressured. There is an air gap between the inside of the enclosure and the outside of the cylinder electrode (see pic) so the electrode can't be heated from the outside.
I've been thinking about adding a pressure sensor and thermocouple between the top valves (see pic) to use the pressure reading as an indicator of liquid phase, instead of opening it from the top. Then, I would release the liquid from the same bottom valve I filled cell from into an empty cylinder with a relatively long pipe in between, to ensure the cooling effect does not reach the cell. Is there anything you think i can do further to reduce the throttling effect in my system? There must be a way of removing the liquid with a gradual decrease in pressure that stops throttling from occurring when opening a valve right?
I was thinking of covering the valves and pipes at the bottom and top of the cell with heat tapes (set to 25C) as well to limit the throttling. The problem with this though is that a temperature gradient could develop maybe causing bubbles to rise to the top of the cell which would affect our reading (we need uniform liquid conditions). Our cell is within a stainless steel cylinder enclosure to hold it together while being pressured. There is an air gap between the inside of the enclosure and the outside of the cylinder electrode (see pic) so the electrode can't be heated from the outside.

![[cry] [cry] [cry]](/data/assets/smilies/cry.gif) ?? I'll definitely talk to the company people about installing a relief valve. Did tell them about the water bath, they were adamant to keep water away from the device to prevent moisture somehow getting in and corrupting the measurement, will tell them again though since that seems unlikely.
?? I'll definitely talk to the company people about installing a relief valve. Did tell them about the water bath, they were adamant to keep water away from the device to prevent moisture somehow getting in and corrupting the measurement, will tell them again though since that seems unlikely.