jangolobow
Chemical
Hay
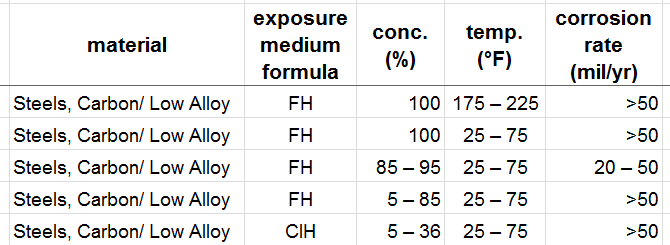
does sombody have some data or info abouth corrosion rate of A352LCC with HF and HCl mixture in any ratio! If not this at least in pure Hf and pure Hcl? temperature abouth 40degC.
Thanks
JG
does sombody have some data or info abouth corrosion rate of A352LCC with HF and HCl mixture in any ratio! If not this at least in pure Hf and pure Hcl? temperature abouth 40degC.
Thanks
JG