Hello fellow engineers. I am looking for a reputable and reliable reference to use when i need a property of a gas. so for example I like to know what is the density of carbon dioxide at 21 Deg. C and 1 bar absolute pressure. I don't want to rely on a page coming up on google search, and I like to have a reputable reference. I came across publication from NIST but I don't know where to buy or get the information.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
-
Congratulations cowski on being selected by the Eng-Tips community for having the most helpful posts in the forums last week. Way to Go!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Gas properties for industrial gasses and air
- Thread starter AlBod
- Start date
- Status
- Not open for further replies.
georgeverghese
Chemical
See page 2-225 in Perry Chem Engg Handbook 7th edition for properties of superheated CO2. If you are not too fussed about accuracy, assume compressibility at 300degK, 1bar abs to be the same as that at 284degK, 1bar abs.
NIST provides a free version miniREFPROP where you can get such information on gas properties.
However AFAIK, that free version would only allow you to check pure compounds (not mixtures) and also it is limited to two or three gases. CO2 is included. I understand CO2 was just an example, so you would like to access virtually any gas, then either you buy the NIST software or use an equation of state (try to google PR, SRK or BWRS and you may come accross some free academic package), but here again often you may not be able to have the full list of properties (example viscosity). If you are about density and the "classic" thermodynamic properties that would work. Another useful resource is the API TDB (technical databook); that is not free by the way.
Edit: Thanks for the link pmover, just added it to my favorites.
However AFAIK, that free version would only allow you to check pure compounds (not mixtures) and also it is limited to two or three gases. CO2 is included. I understand CO2 was just an example, so you would like to access virtually any gas, then either you buy the NIST software or use an equation of state (try to google PR, SRK or BWRS and you may come accross some free academic package), but here again often you may not be able to have the full list of properties (example viscosity). If you are about density and the "classic" thermodynamic properties that would work. Another useful resource is the API TDB (technical databook); that is not free by the way.
Edit: Thanks for the link pmover, just added it to my favorites.
georgeverghese
Chemical
If you dont have access to Perry, density at 294degK / 1bar abs, using z from 300degK/1bar abs, would be 1.810kg/m3
at those conditions (21 C, 1 Bar) a std. EOS can provide good accuracy,
however if you increase pressure you may observe deviations, as example consider the experimental data points for Carbon Dioxide density reported by DDB
herebelow the values for density calculated with a few methods available in REFPROP and PRODE,
T = 290.70 K P = 5066.250 KPa DENS = 149.694 KG/M3
(NIST) REFPROP = 150.65
(Prode) MBWR = 150.9
(Prode) GERG 2008 = 150.6317
(Prode) Lee Kesler Plocker = 152.7519
(Prode) Peng Robinson = 153.6083
T = 292.95 K P = 5572.88 KPa DENS = 181.111 KG/M3
(NIST) REFPROP = 179.99
(Prode) MBWR = 182.3
(Prode) GERG 2008 = 179.99
(Prode) Lee Kesler Plocker = 183.2054
(Prode) Peng Robinson = 183.255
T = 423.15 K P = 27864.4 KPa DENS = 463.263 KG/M3
(NIST) REFPROP = 461.37
(Prode) MBWR = 461.73
(Prode) GERG 2008 = 461.0108
(Prode) Lee Kesler Plocker = 452.8833
(Prode) Peng Robinson = 446.3791
hoping this helps
however if you increase pressure you may observe deviations, as example consider the experimental data points for Carbon Dioxide density reported by DDB
herebelow the values for density calculated with a few methods available in REFPROP and PRODE,
T = 290.70 K P = 5066.250 KPa DENS = 149.694 KG/M3
(NIST) REFPROP = 150.65
(Prode) MBWR = 150.9
(Prode) GERG 2008 = 150.6317
(Prode) Lee Kesler Plocker = 152.7519
(Prode) Peng Robinson = 153.6083
T = 292.95 K P = 5572.88 KPa DENS = 181.111 KG/M3
(NIST) REFPROP = 179.99
(Prode) MBWR = 182.3
(Prode) GERG 2008 = 179.99
(Prode) Lee Kesler Plocker = 183.2054
(Prode) Peng Robinson = 183.255
T = 423.15 K P = 27864.4 KPa DENS = 463.263 KG/M3
(NIST) REFPROP = 461.37
(Prode) MBWR = 461.73
(Prode) GERG 2008 = 461.0108
(Prode) Lee Kesler Plocker = 452.8833
(Prode) Peng Robinson = 446.3791
hoping this helps
Suggest you try Refprop as others have pointed to. Just purchase (make your company purchase) the download.
You can use it with Excel. Lots of good YouTube videos explaining how to run it.
You can use it with Excel. Lots of good YouTube videos explaining how to run it.
apetri,
Just for the fun
I checked in miniREFPROP (free, v9.1) the values are in agreement, there still is some discrepancies...
Native GERG2008 and EOS-CG
CO2 Density kg/m3
150.6307 @ T = 290.70 K ; P = 5066.250 kPa
179.9980 @ T = 292.95 K ; P = 5572.88 kPa
461.0077 @ T = 423.15 K ; P = 27864.4 KPa
NIST miniREFPROP (selecting GERG 2008 model)
CO2 Density kg/m3
150.6307 @ T = 290.70 K ; P = 5066.250 kPa
179.9980 @ T = 292.95 K ; P = 5572.88 kPa
461.0077 @ T = 423.15 K ; P = 27864.4 KPa
Maybe you want to check this (or maybe not).
Hope this helps
Just for the fun
I checked in miniREFPROP (free, v9.1) the values are in agreement, there still is some discrepancies...
Native GERG2008 and EOS-CG
CO2 Density kg/m3
150.6307 @ T = 290.70 K ; P = 5066.250 kPa
179.9980 @ T = 292.95 K ; P = 5572.88 kPa
461.0077 @ T = 423.15 K ; P = 27864.4 KPa
NIST miniREFPROP (selecting GERG 2008 model)
CO2 Density kg/m3
150.6307 @ T = 290.70 K ; P = 5066.250 kPa
179.9980 @ T = 292.95 K ; P = 5572.88 kPa
461.0077 @ T = 423.15 K ; P = 27864.4 KPa
Maybe you want to check this (or maybe not).
Hope this helps
rotw,
yes, I compared GERG 2008 in REFPROP 10 vs. the GERG 2008 / AGA 2017 model available in PRODE Properties 1.2C... ,
there are very little differencies of about 1.0E-6 in densities and other properties,
according the developer these may depend from different constants (PRODE adopts the values published in AGA 2017 standard) and a different solver, REFPROP (based on Fortran code) includes (for derivatives of different properties) the equations provided in the paper while Prode (based on C++ code) adopts different analytical derivatives and different solvers,
of course these small differencies are well below the accuracy of the model,
anyway for those interested to replicate the test, here there is the link to PRODE Properties,
yes, I compared GERG 2008 in REFPROP 10 vs. the GERG 2008 / AGA 2017 model available in PRODE Properties 1.2C... ,
there are very little differencies of about 1.0E-6 in densities and other properties,
according the developer these may depend from different constants (PRODE adopts the values published in AGA 2017 standard) and a different solver, REFPROP (based on Fortran code) includes (for derivatives of different properties) the equations provided in the paper while Prode (based on C++ code) adopts different analytical derivatives and different solvers,
of course these small differencies are well below the accuracy of the model,
anyway for those interested to replicate the test, here there is the link to PRODE Properties,
apetri,
Yes, it is very marginal, agree with you.
One note regarding the source document for the model constants. The ones in the original paper are of course correct (Wagner & al.).
Reason I am mentionning this, is that I found unnaccuracies in the constants published in ISO 20765 Part 2 (edit: 2015).
More specifically, it concerns Appendix B, Table B1 of the standard. I do not know if this has been revised in the newest version of the standard (I guess there is was an update in 2018). This is also a note of caution to others in case they are working with these standards.
Yes, it is very marginal, agree with you.
One note regarding the source document for the model constants. The ones in the original paper are of course correct (Wagner & al.).
Reason I am mentionning this, is that I found unnaccuracies in the constants published in ISO 20765 Part 2 (edit: 2015).
More specifically, it concerns Appendix B, Table B1 of the standard. I do not know if this has been revised in the newest version of the standard (I guess there is was an update in 2018). This is also a note of caution to others in case they are working with these standards.
I just searched a bit more in my archive about this point.
It seems at least there is one mismatch I spotted.
It is for CO2 when conparing between original paper (GERG2004) and ISO standard 20765-Part II (GERG expansion 2004 -> 2008 there was no supposed change regarding the coefficients for CO2 in Table B-1). So here it is:
ISO20765 Part2
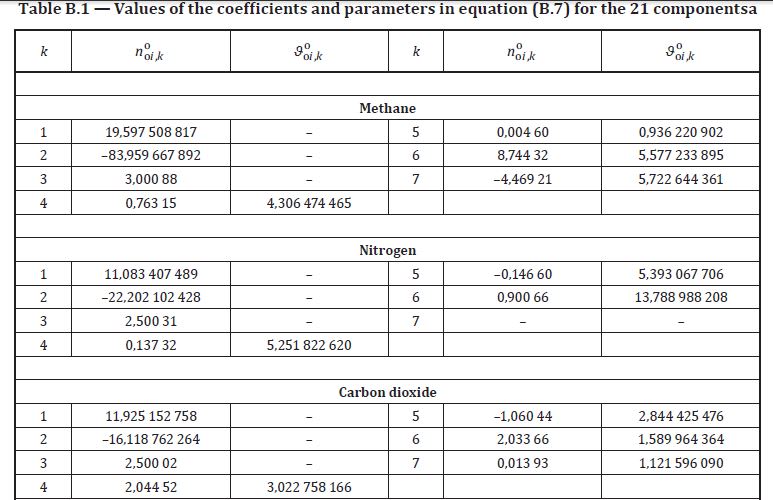
Original Paper
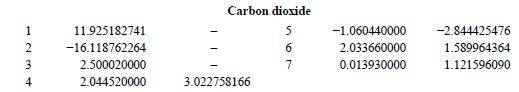
Edit: same type of mistmatch for Nitrogen too...
It seems at least there is one mismatch I spotted.
It is for CO2 when conparing between original paper (GERG2004) and ISO standard 20765-Part II (GERG expansion 2004 -> 2008 there was no supposed change regarding the coefficients for CO2 in Table B-1). So here it is:
ISO20765 Part2

Original Paper
Edit: same type of mistmatch for Nitrogen too...
- Status
- Not open for further replies.
Similar threads
- Replies
- 10
- Views
- 3K
- Question
- Replies
- 1
- Views
- 2K
- Replies
- 1
- Views
- 736
- Locked
- Question
- Replies
- 7
- Views
- 1K
- Locked
- Question
- Replies
- 3
- Views
- 3K

![[bluegreedy] [bluegreedy] [bluegreedy]](/data/assets/smilies/bluegreedy.gif)