Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
-
Congratulations MintJulep on being selected by the Eng-Tips community for having the most helpful posts in the forums last week. Way to Go!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Is Galvanic Corrosion related to Cathode & Anode Corrosion? 2
- Thread starter Lovison
- Start date
- Status
- Not open for further replies.
-
1
- #2
Wayne:
The anode and cathode are just components of a galvanic cell. Check this site out:
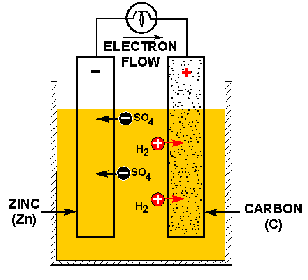
In this example, the zinc plate is the anode and the copper plate is the cathode.
Let me know if you need more info.
Good luck!
jproj
The anode and cathode are just components of a galvanic cell. Check this site out:
In this example, the zinc plate is the anode and the copper plate is the cathode.
Let me know if you need more info.
Good luck!
jproj
- Thread starter
- #3
- Thread starter
- #5
jporj,
You've out done yourself. Nice Job. I take it that in order to a have galvanic reaction or occurrence one or more incompatible materials must be present to cause a reaction such as we've discussed. Like materials are not affected where as others being more noble or less noble would start the process.
Is it common to see sacrificial components being used to protect other parts? Would salt water be a good case for this sacrificial element?
Is a galvanic corrosion an oversite missed by the systems process engineer or does it occur from some field modification or material substitution? Where is galvanic corrosion normally found and is it done on purpose by any reason?
Wayne E. Lovison
service-parts@naglepumps.com
You've out done yourself. Nice Job. I take it that in order to a have galvanic reaction or occurrence one or more incompatible materials must be present to cause a reaction such as we've discussed. Like materials are not affected where as others being more noble or less noble would start the process.
Is it common to see sacrificial components being used to protect other parts? Would salt water be a good case for this sacrificial element?
Is a galvanic corrosion an oversite missed by the systems process engineer or does it occur from some field modification or material substitution? Where is galvanic corrosion normally found and is it done on purpose by any reason?
Wayne E. Lovison
service-parts@naglepumps.com
Wayne:
I work in equipment design (mainly boiler feedwater components) and ocasionally see customer specifications requiring sacrificial andode to protect other (vital) components.
As you have noticed, salt water and aluminum do not mix well. Aluminum has a protective oxide layer that generally protects the metal from further corrosion. Chlorine ions (Cl-) from salt water (NaCl) can penetrate this protective oxide layer and promote corrosion of the underlying metal. (I read somewhere that San Fransisco doesn't use aluminum light poles for this reason).
It seams like you should use a different material (Stainless Steel if that is working in other installations.) I would also caution that stainless steel is susceptable to corrosion from chlorine as well.
I basically design equipment in contact with water or steam with a low oxygen presence and normally don't have much dealing with corrosion design. Material selection is an important part of any piece of equipment, though, and should be taken care of in the design phase. Field modification is often done without complete knowlege of the design (i.e. why certain things were specified the way they were)and leads to problems.
The most recognizable instance of galvanic corrosion is in a battery. There are other instances where galvanic corrosion takes place that are both good (on purpose) and bad. Using a sacrificial anode to protect a vital component is an example of a purposeful use of galvanic corrosion. I'm sure you can think of plenty bad cases.
Hope this helps!
jproj
I work in equipment design (mainly boiler feedwater components) and ocasionally see customer specifications requiring sacrificial andode to protect other (vital) components.
As you have noticed, salt water and aluminum do not mix well. Aluminum has a protective oxide layer that generally protects the metal from further corrosion. Chlorine ions (Cl-) from salt water (NaCl) can penetrate this protective oxide layer and promote corrosion of the underlying metal. (I read somewhere that San Fransisco doesn't use aluminum light poles for this reason).
It seams like you should use a different material (Stainless Steel if that is working in other installations.) I would also caution that stainless steel is susceptable to corrosion from chlorine as well.
I basically design equipment in contact with water or steam with a low oxygen presence and normally don't have much dealing with corrosion design. Material selection is an important part of any piece of equipment, though, and should be taken care of in the design phase. Field modification is often done without complete knowlege of the design (i.e. why certain things were specified the way they were)and leads to problems.
The most recognizable instance of galvanic corrosion is in a battery. There are other instances where galvanic corrosion takes place that are both good (on purpose) and bad. Using a sacrificial anode to protect a vital component is an example of a purposeful use of galvanic corrosion. I'm sure you can think of plenty bad cases.
Hope this helps!
jproj
-
1
- #7
Frequently zinc is used as a sacrifical anode for ships. They can be quite large, in fact.
For your situation, get a copy of the electrovalant chart and select an appropriate material to protect your substrate.
Crashj 'just like a battery' Johnson
For your situation, get a copy of the electrovalant chart and select an appropriate material to protect your substrate.
Crashj 'just like a battery' Johnson
- Status
- Not open for further replies.
Similar threads
- Replies
- 17
- Views
- 27K
- Replies
- 4
- Views
- 3K
- Replies
- 7
- Views
- 3K
- Replies
- 5
- Views
- 1K
- Replies
- 1
- Views
- 539