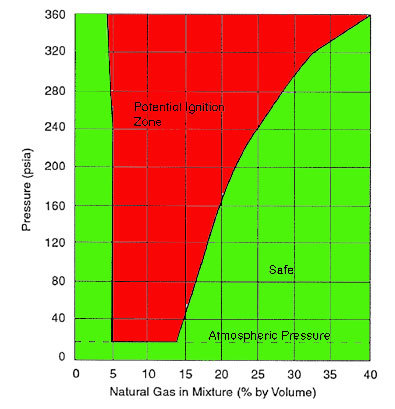
The Upper Explosive Limit for methane in air at atmospheric pressure is 15% (150,000 ppm) (slightly lower at lower pressures). That would say that the oxygen number is 17% (170,000 ppm)for the methane UEL (remember, you are approaching the chart below from the right, not the left)
Pipeline specs used to be around 2,000 ppm, but there is a lot of pressure from the Nanny Society to lower that number to 15 ppm. The pipeline spec is all about the risk of oxygen-related corrosion modalities, not combustion.
Since you need 6 times as much air as you have methane to support combustion, a VRU designed to extract 1 MSCF/day of gas from a tank (big VRU) would have to be able to compress 7 MSCF/day to get in the explosive range. I don't know about you, but I don't size compression to move 7 times my target volume.
I have done a lot of research for the last 20 years or so (since I investigated a fatality related to improper purging), and I cannot find a single occasion of an air-ingestion related explosion or fire in a compressor. I find a lot of compressor fires, but they are all either related to improper purging of the suction lines (i.e., compressing air until the machine gets hot and then suck in a cylinder full of a fuel/air mixture) or a vent line blowing on a hot element.
Mostly the instruments I see in vacuum service in upstream are 0-500 ppm or 0-5000 ppm. I've used several brands and they seem to be similar to each other. Some require more purge gas, some less. Some tolerate liquid better than others. The devices today seem to be a lot closer to each other in performance (all tend to be very good to excellent) than just 10 years ago when I deployed the first one I ever saw in the field (and it was really delicate, a drop of liquid would shut it down for days). I can't tell you what the P&ID symbol is.
David Simpson, PE
MuleShoe Engineering
In questions of science, the authority of a thousand is not worth the humble reasoning of a single individual. Galileo Galilei, Italian Physicist