helpyou
Automotive
- Sep 26, 2011
- 79
HI
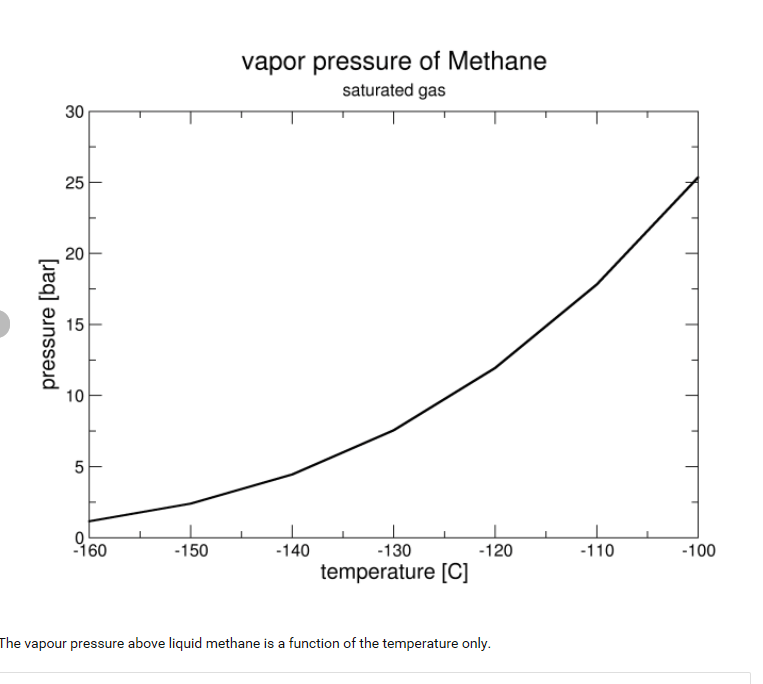
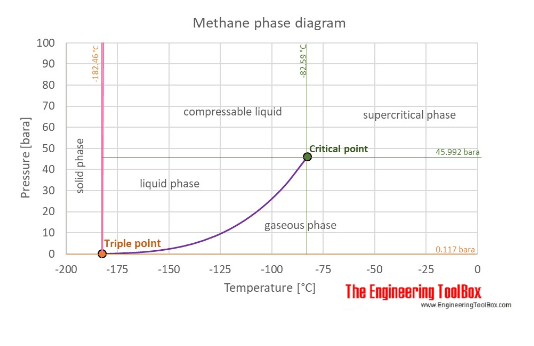
on an LNG (liquified natural gas) pipe, I understand that there is LNG vapor pressure with the Liquid. Is the LNG vapor pressure equal to the LNG pressure (Liquid pressure)? Reason is, there are pressure gauges mounted on top of pipe and reading pressure. I assume they are reading vapor pressure. Can someone clarify this point from experience please?
Thank you
on an LNG (liquified natural gas) pipe, I understand that there is LNG vapor pressure with the Liquid. Is the LNG vapor pressure equal to the LNG pressure (Liquid pressure)? Reason is, there are pressure gauges mounted on top of pipe and reading pressure. I assume they are reading vapor pressure. Can someone clarify this point from experience please?
Thank you