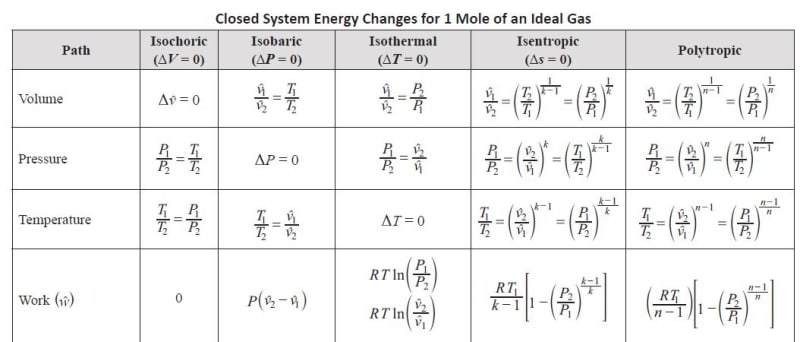
In a closed system the work on compressing a non-flowing fluid is the integral of (P dV) = Force x Distance = P*A*dX
Where PVk = Constant or P = C/Vk
So the integral is (C/Vk)dV - The solution to this integral is the equation for closed flow in your table.
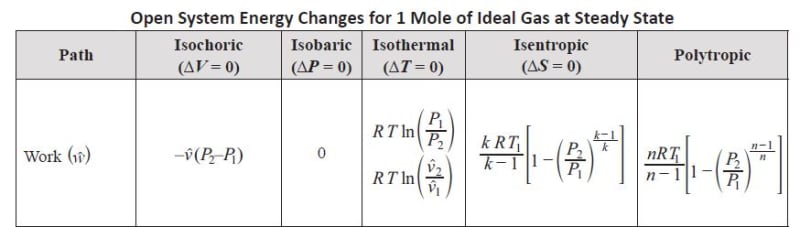
In an open system the work is the integral of (V dP). This can be seen by the steady flow energy equation as follows:
TdS - W = dU + d(PV) + dKE + dPE = dU + PdV +VdP + dKE + dPE
But in a non-flow closed system we know that any heat added or removed Q = TdS = dU + PdV which is also true for a flowing system - so in a process with no heat added process such as an isentropic compression as the pressure increases and the dU increases due to compression, the integral of PdV is negative, because V2 is smaller than V1, but of equal value as positive dU so they all cancel - so PdV is still the pure compression work (that goes into dU) but VdP is the total work of compression and pushing the gas out of the cylinder once the compression has been completed, and pulling new gas back into the cylinder for compression - note that PdV is called the area under the curve and VdP is called the area behind the curve in thermo books.
so TdS and dU + PdV cancels out of the above equation for steady flow and the resulting steady flow equation is:
VdP = dKE + dPE + W
Typically change in kinetic energy and potential energy are negligible so the equation reduces to:
Integral of VdP = Work Steady Flow
The soliution of this integral is the equation shown for an open system in your table.