Em_And_Chem
Student
I can't seem to get the right answer, any help is appreciated!
I have a question under the topic "First law of thermodynamics", I've also been told that I should use the complete Energy (or perhaps entropy?) balances.
The question goes as follows; A body of iron with constant heat capacity 460 J/(kg K) and mass 3 kg is heated with a heating effect of 500 W. How long does it take to heat the body from 250C to 1000C if the heat loss to the surroundings can be estimated at 15 W?
so far Ive (perhaps incorrectly) assumed:
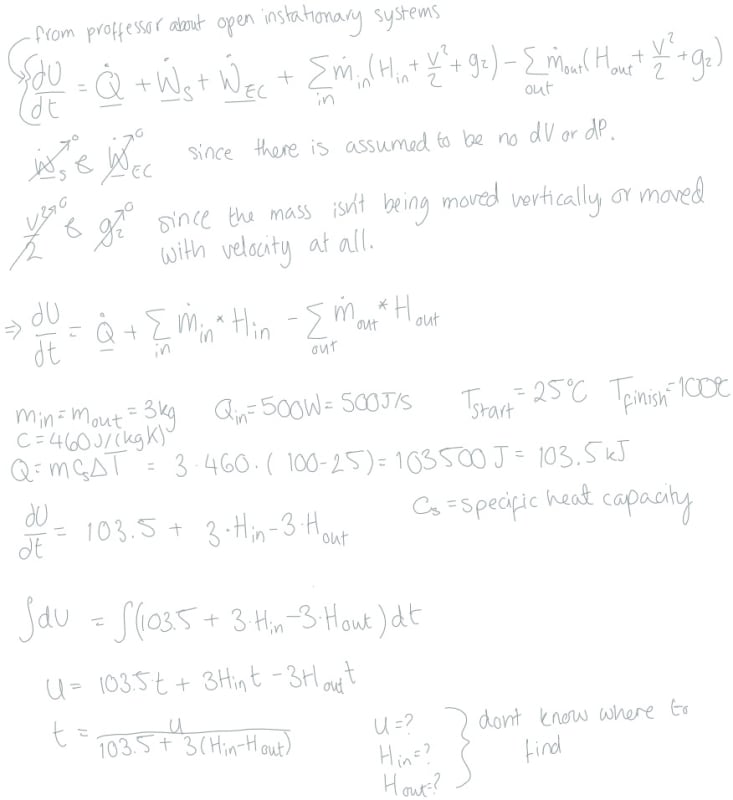
The answer is apparently 213 seconds
I have a question under the topic "First law of thermodynamics", I've also been told that I should use the complete Energy (or perhaps entropy?) balances.
The question goes as follows; A body of iron with constant heat capacity 460 J/(kg K) and mass 3 kg is heated with a heating effect of 500 W. How long does it take to heat the body from 250C to 1000C if the heat loss to the surroundings can be estimated at 15 W?
so far Ive (perhaps incorrectly) assumed:
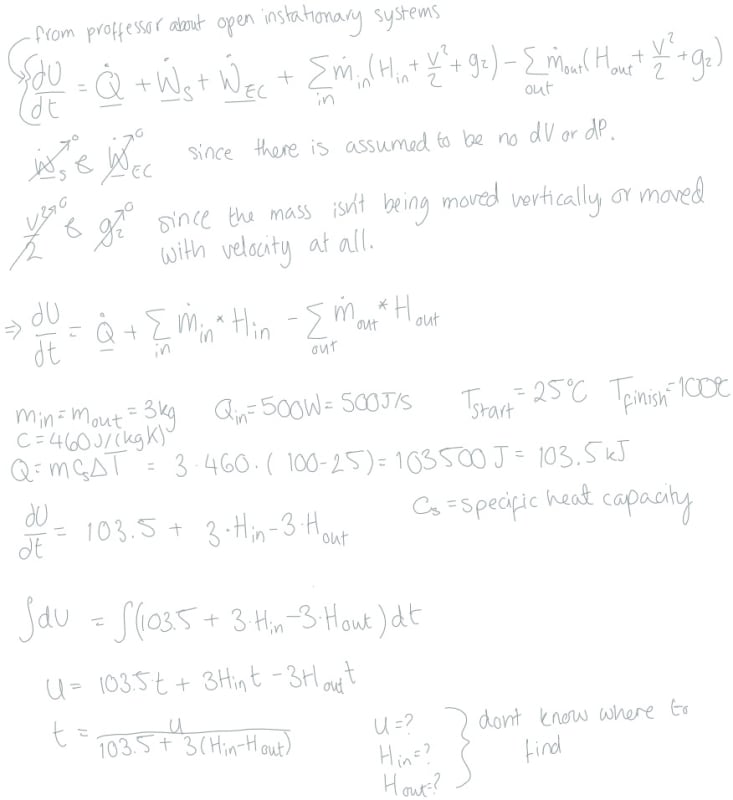
The answer is apparently 213 seconds
