rQuestionEngineering
Mechanical
I am trying to calculated power requirements from the below data.
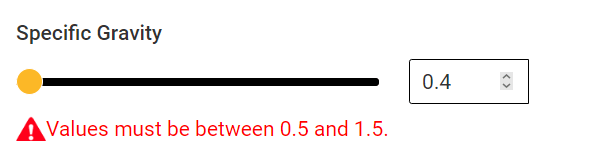
However , I am not able to enter data specific gravity below 0.5
The gas molecular weight given to me is 11kg/kmol.
What is the specific gravity ?
However , I am not able to enter data specific gravity below 0.5
The gas molecular weight given to me is 11kg/kmol.
What is the specific gravity ?

