WPPM - "weight parts per million" is a reasonable guess, since I'm pretty sure you don't mean "white people party music" or "windsor pacific property management".
"WPPM" is a measurement of concentration on a weight or volume basis. Parts per million by mass is equivalent to milligrams per liter (mg/L). Parts per million by volume is a common way to measure concentrations of gases.
The molarity of a solution is the number of moles of solute per liter of solution. The molar mass of any particle (atom, molecule, formula, or ion) is the sum of the average atomic masses of all atoms forming that particle. This is also known as molecular weight. The molar mass of an atom is its average atomic mass expressed in grams. The atomic mass of all atoms can be found in the periodic table. For dilute solutions, one part per million equals one mg/L. In most cases, the two are considered equal.
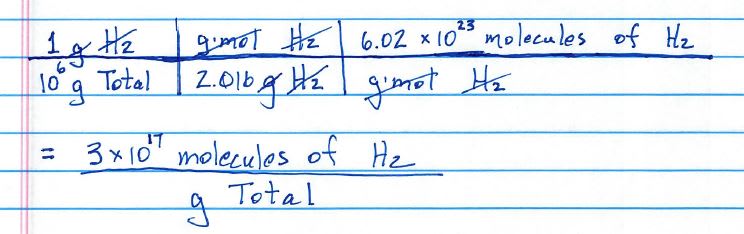
To convert from molarity to ppm, first determine the molar mass of a substance. For instance, hydrogen (H2) has a molar mass of 2x1.00794 = 2.01588. A solution with 1 M concentration would have 2.01588 g of hydrogen gas (H2) per 1 L of solution. As a gas, there would be 2.016 g of H2 in something with a total mass of one million (1E6) grams.
Converting energy to motion for more than half a century