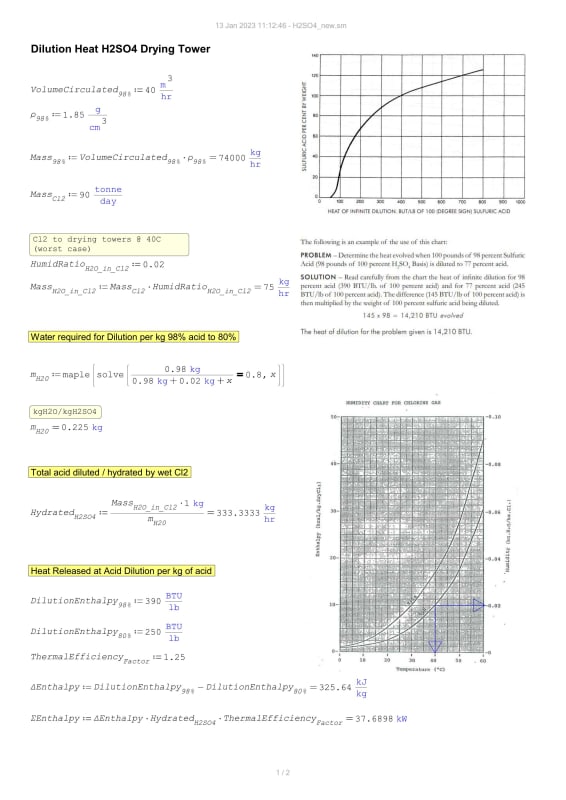
I am unable to find a suitable value for Heat of Dilution of concentrated H2SO4. I will share my calculation here as well but first I need this value to be settled.
I have x2 documents
1] 95 kJ/mol 2] 71.76 kJ/mol
Background: I am determining cooling load while drying wet Cl2 gas using circulated H2SO4 in a drying packed tower (98w.t% > 78w.t%)
I have x2 documents
1] 95 kJ/mol 2] 71.76 kJ/mol
Background: I am determining cooling load while drying wet Cl2 gas using circulated H2SO4 in a drying packed tower (98w.t% > 78w.t%)