VFreshEngineer
Mechanical
Hi all,
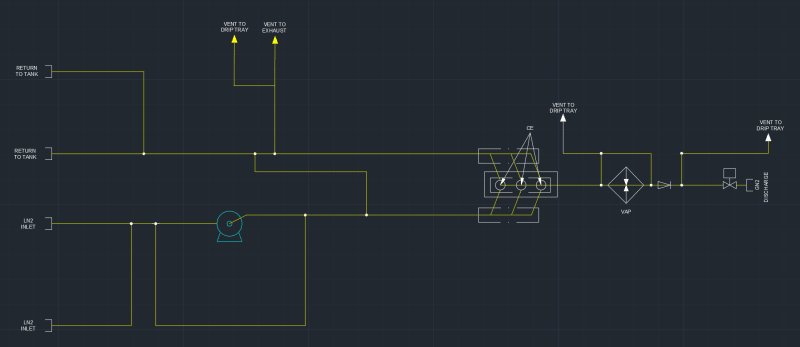
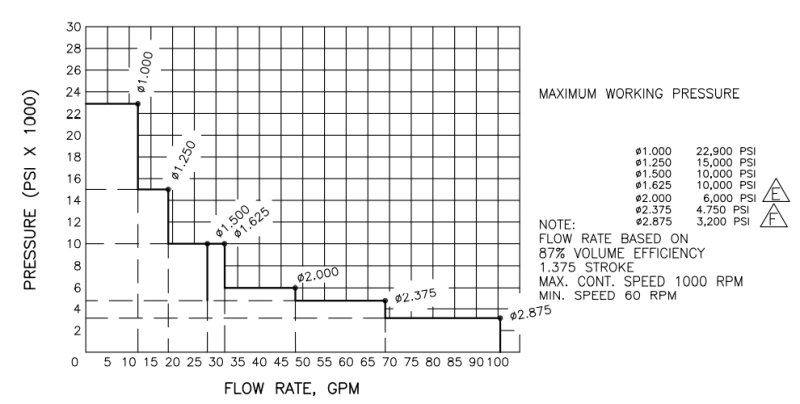
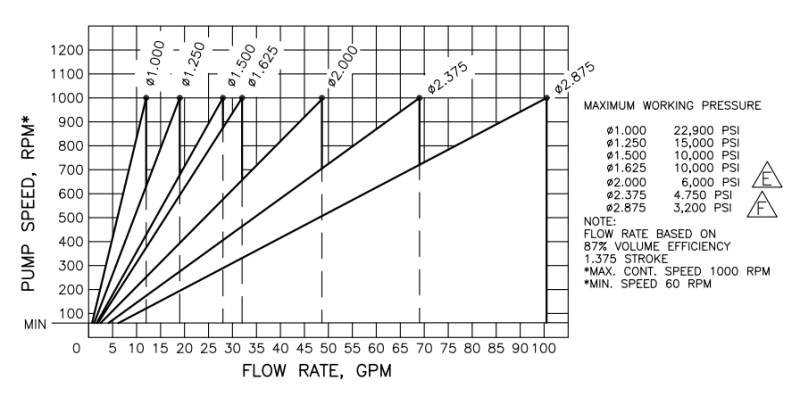
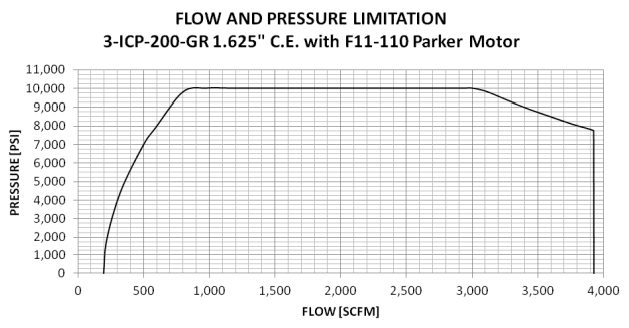
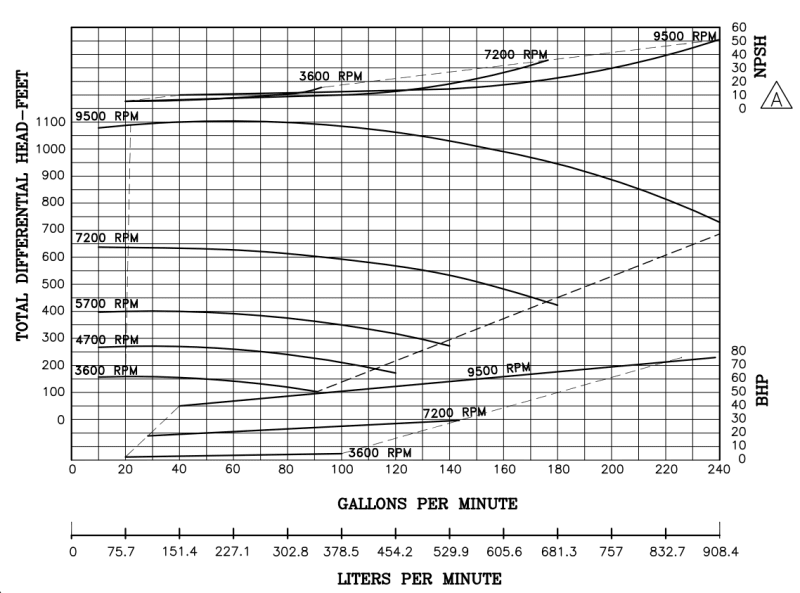
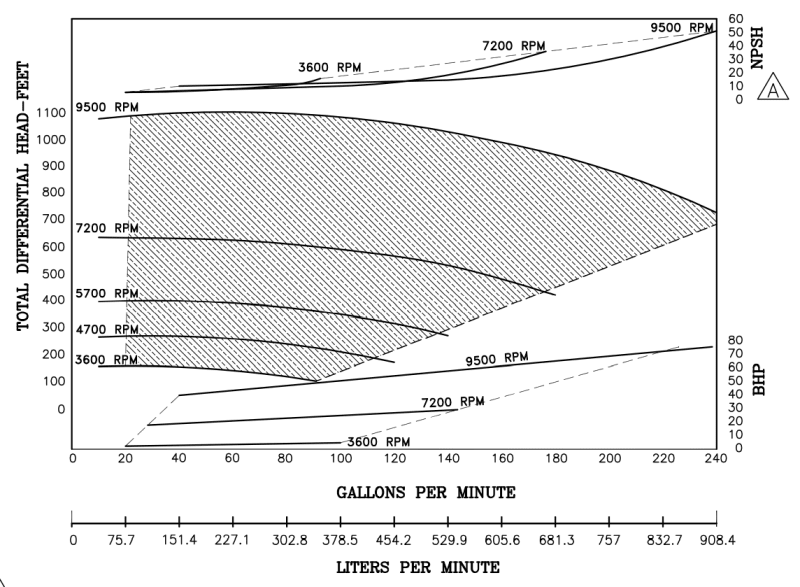
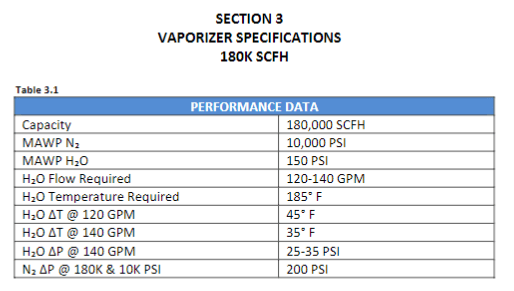
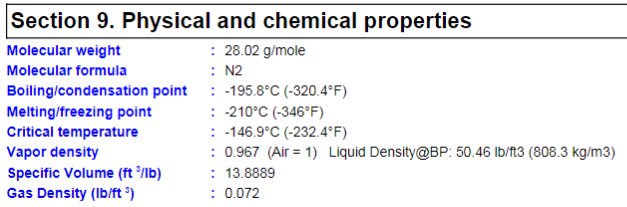
I'm a fresh mechanical engineer with no others in my company, so I don't really have anyone to discuss stuff with. I don't have much experience with thermodynamics, so I would really appreciate all the help I can get. I have to provide the minimum discharge temperature when at the highest rate and lowest pressure going through vaporizer. The vaporized medium will be Nitrogen. The pump specifications are as follows:
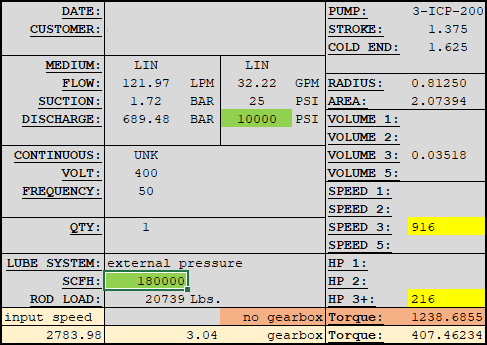
Any ideas how to approach this?
I'm a fresh mechanical engineer with no others in my company, so I don't really have anyone to discuss stuff with. I don't have much experience with thermodynamics, so I would really appreciate all the help I can get. I have to provide the minimum discharge temperature when at the highest rate and lowest pressure going through vaporizer. The vaporized medium will be Nitrogen. The pump specifications are as follows:

Any ideas how to approach this?