Hello, sorry if this question is dumb, but why it is difficult to use petrol in engine which is like diesel? What if petrol would be injected into hot air near tdc using high pressure fuel pump and direct injection, also higer cr engine, like in diesel engines? I saw, that few years ago, Hyundai and Delphi was working on project like this.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
-
Congratulations MintJulep on being selected by the Eng-Tips community for having the most helpful posts in the forums last week. Way to Go!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
gasoline direct injection compression ignition engine
- Thread starter Deividas
- Start date
- Status
- Not open for further replies.
you would need a much higher compression ratio to actually reach the auto-ignition temperature when using gasoline. however, when that temperature would be reached, combustion might start at various locations within the combustion chamber in a more or less random order, which could result in localized pressure peaks, "rough combustion", overheating and less efficiency.
dieselfuel will start to burn more evenly once the auto-ignition temperature is reached - and the auto-ignition temperature is substantially lower, due to the chemical structure of the components that make up the dieselfuel (relatively long and straight hydrocarbons compared with relatively short predominantly branched hydrocarbons for gasoline).
dieselfuel will start to burn more evenly once the auto-ignition temperature is reached - and the auto-ignition temperature is substantially lower, due to the chemical structure of the components that make up the dieselfuel (relatively long and straight hydrocarbons compared with relatively short predominantly branched hydrocarbons for gasoline).
RadiusOfTheCircle
Automotive
I wondered the same thing myself and found an interesting study of just such an experimental engine on google. The author of the study believed that using fuels like kerosine which are less refined than petrol solves the octane problem romke mentions. Please note that this was not an HCCI engine, it injects the fuel like a diesel engine towards the end of the compression stroke, so it seems to me this engine would have combustion similar to that of a diesel.
GregLocock
Automotive
Kerosene is pretty much the light end of diesel, so he seems to be using a diesel like fuel in a diesel like engine. Wow.
Cheers
Greg Locock
New here? Try reading these, they might help FAQ731-376
Cheers
Greg Locock
New here? Try reading these, they might help FAQ731-376
BrianPetersen
Mechanical
Well, if you put gasoline into the fuel tank of a diesel engine, engine no workie. Plenty of people have found that out accidentally over the years - by filling the fuel tank of a diesel-powered vehicle with gasoline.
Diesel fuel is designed to ignite easily with minimal delay when squirted into hot air. Gasoline is designed NOT to ignite easily (i.e. have a rather long ignition delay) when compressed in hot air (that's called "detonation", and it breaks stuff) but ignite easily with a spark (it vaporises much more easily than diesel fuel does). So, if you squirt gasoline into the injection system of a diesel engine ... it either doesn't work at all, or it kindasorta works under some conditions but the engine is quite obviously not happy about it.
Mazda's upcoming "Skyactiv X" engine is worthy of study. It's using premixed air and fuel charge with a lot of EGR ... and spark ignition to light off the charge. The balance of the charge basically detonates intentionally.
So with that, what's your question?
Diesel fuel is designed to ignite easily with minimal delay when squirted into hot air. Gasoline is designed NOT to ignite easily (i.e. have a rather long ignition delay) when compressed in hot air (that's called "detonation", and it breaks stuff) but ignite easily with a spark (it vaporises much more easily than diesel fuel does). So, if you squirt gasoline into the injection system of a diesel engine ... it either doesn't work at all, or it kindasorta works under some conditions but the engine is quite obviously not happy about it.
Mazda's upcoming "Skyactiv X" engine is worthy of study. It's using premixed air and fuel charge with a lot of EGR ... and spark ignition to light off the charge. The balance of the charge basically detonates intentionally.
So with that, what's your question?
RadiusOfTheCircle
Automotive
GregLocock - Admittedly I was taking a bit of a stab in the dark when I said kerosine as it's been a while since I read the paper but IMS he believed many less refined fuels could run in his engine. It was also his belief that should his engine become widely adopted substantial savings would be made on petrol refinement due to the icreased usability of less refined fuels.
BP - petrol does indeed "workie" in a diesel car (note this in the video) - in fact it probably "workies" a bit better than diesel fuel does. But it will certainly bugger the injector pump etc. fairly quickly. It is common practice in the Australian bush to tip a bit of petrol directly into the intake of a diesel engine if it is reluctant to start.
- Thread starter
- #9
i know that petrol is not good lubricant compared to diesel, but i think this is not the biggest problem in this situation, there are many direct injection petrol engines with high pressure fuel pumps, also you can use lower octane petrol or higher cr engine to ensure ignition near tdc
enginesrus
Mechanical
It is nothing new. It has and is done, there have been many multi fuel diesel engines that have been built, and yes since the self ignition quality's of diesel vs gasoline are inverse, meaning diesel is easy to ignite under compression pressure / heat, gasoline is not, octane resists that sort of combustion, so then higher pressures are needed.
If I remember the Detroit multifuel engine was a 21:1 compression ratio. A small amount of oil can be added like for a 2 stroke chainsaw engine to gasoline for more lubricity. But then how many electronic and now electronic direct gasoline injection systems are in operation? And in the old days how many mechanical gasoline injection systems were used in large aircraft engines, and some higher end cars? Mercedes had an engine that used mechanical injection back in the day.
So yes a diesel will burn gasoline just fine with the correct modifications, a low cr emissions designed one will not do so good.
If I remember the Detroit multifuel engine was a 21:1 compression ratio. A small amount of oil can be added like for a 2 stroke chainsaw engine to gasoline for more lubricity. But then how many electronic and now electronic direct gasoline injection systems are in operation? And in the old days how many mechanical gasoline injection systems were used in large aircraft engines, and some higher end cars? Mercedes had an engine that used mechanical injection back in the day.
So yes a diesel will burn gasoline just fine with the correct modifications, a low cr emissions designed one will not do so good.
Current gasoline direct injection pressures are far lower than diesel injection pressures and from what I understand the high pressure GDI pumps still have a relatively high failure rate. The old mechanical GDI systems were much lower pressure.
----------------------------------------
The Help for this program was created in Windows Help format, which depends on a feature that isn't included in this version of Windows.
----------------------------------------
The Help for this program was created in Windows Help format, which depends on a feature that isn't included in this version of Windows.
enginesareus,
I believe "since the self ignition quality's of diesel vs gasoline are inverse, meaning diesel is easy to ignite under compression pressure / heat, gasoline is not, octane resists that sort of combustion, so then higher pressures are needed" is incorrect. High and low octain fuels both begin combustion at roughly similar pressures and temperatures. The key factor, as BrianPeterson notes, is ignition delay.
The characteristics of heptane (0 octane) and iso-octane (100 octane) under Homogeneous Charge Compression Ignition (HCCI) are shown below (note HCCI is not the same as the diesel or spark ignition process but often uses one or the other fuel). Diesel #2 is around PRF 62 while most gasoline is near PRF90. The figure shows similar pressures and temperatures for a given ignition point, but significantly different ignition delay.
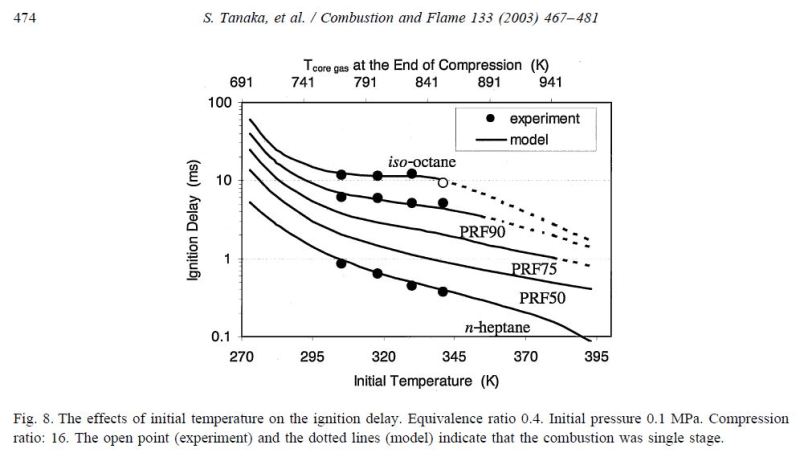
This figure from the same paper provides more insight into what's happening during compression ignition. Note that all the reference fuels begin ignition near 810K and pass through a period of low temperature heat release then, at around 1100K, begin high temperature heat release when pressure begins a steep climb. After this point, the pressure slopes of the different fuels are similar.
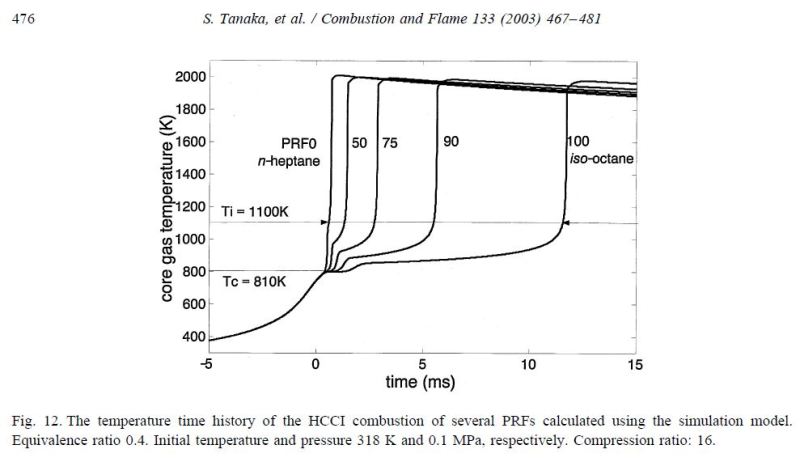
From the figures, it's clear why spark ignition engines use high octane while diesel engines use low octane fuel; spark ignition engines using gasoline exploit the increased ignition delay of high octane fuels to allow more compression before high temperature heat release while diesel engines use low octane so bulk heat release occurs rapidly upon injection of fuel into the pre-heated air.
P.S. Note time-zero associated with ignition delay in the top figure begins when piston acceleration changes sign. This trigger was selected purely for convenience and reflects behavior of the mechanical apparatus used. Per the paper, the time from change of piston acceleration sign to combustion is 0.4 ms, so ignition delays of the figures should be reduced by that time period to remove that bias.
I believe "since the self ignition quality's of diesel vs gasoline are inverse, meaning diesel is easy to ignite under compression pressure / heat, gasoline is not, octane resists that sort of combustion, so then higher pressures are needed" is incorrect. High and low octain fuels both begin combustion at roughly similar pressures and temperatures. The key factor, as BrianPeterson notes, is ignition delay.
The characteristics of heptane (0 octane) and iso-octane (100 octane) under Homogeneous Charge Compression Ignition (HCCI) are shown below (note HCCI is not the same as the diesel or spark ignition process but often uses one or the other fuel). Diesel #2 is around PRF 62 while most gasoline is near PRF90. The figure shows similar pressures and temperatures for a given ignition point, but significantly different ignition delay.

This figure from the same paper provides more insight into what's happening during compression ignition. Note that all the reference fuels begin ignition near 810K and pass through a period of low temperature heat release then, at around 1100K, begin high temperature heat release when pressure begins a steep climb. After this point, the pressure slopes of the different fuels are similar.

From the figures, it's clear why spark ignition engines use high octane while diesel engines use low octane fuel; spark ignition engines using gasoline exploit the increased ignition delay of high octane fuels to allow more compression before high temperature heat release while diesel engines use low octane so bulk heat release occurs rapidly upon injection of fuel into the pre-heated air.
P.S. Note time-zero associated with ignition delay in the top figure begins when piston acceleration changes sign. This trigger was selected purely for convenience and reflects behavior of the mechanical apparatus used. Per the paper, the time from change of piston acceleration sign to combustion is 0.4 ms, so ignition delays of the figures should be reduced by that time period to remove that bias.
BrianPetersen
Mechanical
To put the above diagrams into perspective, 16:1 is a rather high compression ratio for a gasoline engine but on the low side for a diesel.
1 millisecond of ignition delay at 3000 rpm is 18 crank degrees. It's pretty plausible for n-heptane to self-ignite within that time period so it's pretty likely that a compression-ignition engine would run fine on that. But 10 milliseconds of ignition delay for iso-octane is 180 crank degrees at 3000 rpm ... misfire.
"But the diesel engine in the youtube video kept running when they put gasoline in it" ...
Keep in mind ...
They didn't purge the entire fuel system of diesel. They were only trying to simulate someone accidentally putting gasoline into the tank - also not purging the entire fuel system. In the combustion chamber, the remaining proportion of heavier-molecule diesel in the fuel mixture would help light off the gasoline.
They did it with the engine hot. They never showed us a cold-start attempt.
That was an older car ... not one with high-tech common-rail injection. The old prechamber diesels were more tolerant of off-spec fuel.
1 millisecond of ignition delay at 3000 rpm is 18 crank degrees. It's pretty plausible for n-heptane to self-ignite within that time period so it's pretty likely that a compression-ignition engine would run fine on that. But 10 milliseconds of ignition delay for iso-octane is 180 crank degrees at 3000 rpm ... misfire.
"But the diesel engine in the youtube video kept running when they put gasoline in it" ...
Keep in mind ...
They didn't purge the entire fuel system of diesel. They were only trying to simulate someone accidentally putting gasoline into the tank - also not purging the entire fuel system. In the combustion chamber, the remaining proportion of heavier-molecule diesel in the fuel mixture would help light off the gasoline.
They did it with the engine hot. They never showed us a cold-start attempt.
That was an older car ... not one with high-tech common-rail injection. The old prechamber diesels were more tolerant of off-spec fuel.
enginesrus
Mechanical
Rod, what ever,, what I said about inverse is true, who cares what the cause is, the statement is correct.
You really need to study what high octane rating in a gasoline engine is for and what it does, that validates what I said.
You really need to study what high octane rating in a gasoline engine is for and what it does, that validates what I said.
- Status
- Not open for further replies.
Similar threads
- Question
- Replies
- 33
- Views
- 6K
- Replies
- 10
- Views
- 11K
- Replies
- 5
- Views
- 867
- Replies
- 27
- Views
- 13K
