maxnystrom
Chemical
- Mar 14, 2017
- 4
Hello!
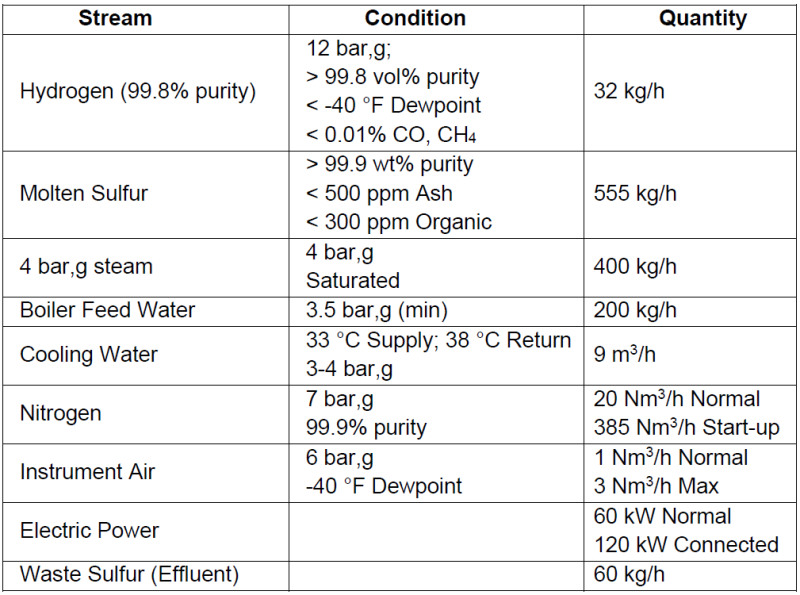
I am new to this forum and was wondering if anyone has any good resources for the production of H2S from molten sulfur and hydrogen. All the resources I find refer to the treatment of H2S or the conversion of H2S to hydrogen and elemental sulfur. I am looking for the capital costs or installation cost for the process together with the capacity. I know it is a bit unusual but would need the H2S for another process. Many thanks!
I am new to this forum and was wondering if anyone has any good resources for the production of H2S from molten sulfur and hydrogen. All the resources I find refer to the treatment of H2S or the conversion of H2S to hydrogen and elemental sulfur. I am looking for the capital costs or installation cost for the process together with the capacity. I know it is a bit unusual but would need the H2S for another process. Many thanks!