RWerth
Chemical
- Apr 10, 2019
- 3
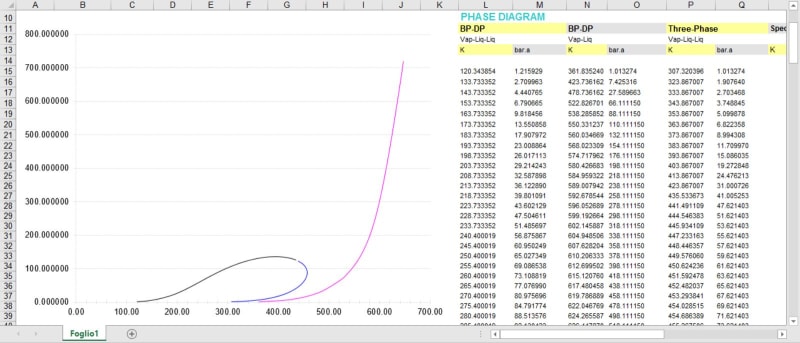
I am trying to size a PRV for the fire case on a three phase (oil/water/gas) using API 521. We have a customer with an oil stream whose concentrations are fairly well dispersed (C1-C10+), and it can be assumed by the separator design that the vessel will maintain something close to a 50/50 water/oil split. The customer has supplied a laboratory breakdown of the components in the oil and to my surprise also included a phase diagram relating the partial pressure of the oil mixture to the temperature of the system. My immediate thought was Clausius-Clapeyron to determine the heat of vaporization, but upon performing this calculation I am getting heat of vaporization values approximately 7.5 X smaller than the typical value used (150 Btu/lb). I believe this is due to the fact that we are using the total wetted surface area, but are not including the water in the heat of vaporization calculation. Important considerations:
* My company doesn't typically get into process simulation and as a result I have no access to commercial sim software.
* The customer is happy to upsize, and in current system, it would not be a large change as the system operates at high P. The same can not be said for future situations, and I am trying to establish a precedent.
* I am hoping to operate within RAGAGEP (of course) but also don't know if it is practical to assume the whole vessel is filled with oil and use the previously described Hvap. Again, I would like to establish a standard method here, but an unrealistic result could both oversize the PRV and get shot down by the people in charge of purchasing.
My questions:
* From the documents we have received from engineering firms, it seems as though most use a value of approximately 150 Btu/lb. This seems to be an industry standard, but I would like to understand if/why it applies instead of just taking it at face value.
* Are there any other good engineering practices to estimate the heat of vaporization? It seems like I keep circling back to thermodynamics, but the more I investigate, the deeper the rabbit-hole goes. I'm happy to continue this pursuit, but I would prefer to know that it is the only option before building my own thermo flash-calculation simulator .
.
Thank you for your time,
RWerth
* My company doesn't typically get into process simulation and as a result I have no access to commercial sim software.
* The customer is happy to upsize, and in current system, it would not be a large change as the system operates at high P. The same can not be said for future situations, and I am trying to establish a precedent.
* I am hoping to operate within RAGAGEP (of course) but also don't know if it is practical to assume the whole vessel is filled with oil and use the previously described Hvap. Again, I would like to establish a standard method here, but an unrealistic result could both oversize the PRV and get shot down by the people in charge of purchasing.
My questions:
* From the documents we have received from engineering firms, it seems as though most use a value of approximately 150 Btu/lb. This seems to be an industry standard, but I would like to understand if/why it applies instead of just taking it at face value.
* Are there any other good engineering practices to estimate the heat of vaporization? It seems like I keep circling back to thermodynamics, but the more I investigate, the deeper the rabbit-hole goes. I'm happy to continue this pursuit, but I would prefer to know that it is the only option before building my own thermo flash-calculation simulator
Thank you for your time,
RWerth