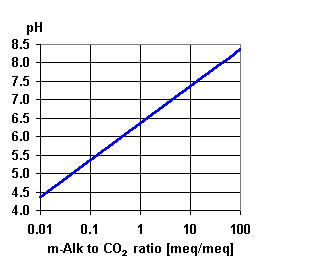
It will depend on the chemistry of the water that you are treating and you have not given much information.
But typically its caused by the carbon dioxide in solution. Two things happen to cause the pH drop.
The CO2 already in solution in the feed water will pass straight through the membrane into the permeate or product water with very little going out with the concentrate. Secondly the RO process removes most of the alkalinity which buffers the acidic effect of the CO2 in solution which is in the form of carbonic acid. With less alkalinity to buffer the carbonic acid the pH drops.
This typically happens where the feed water is less than about 8.0 ph. Plants that have feedwater pHs above 8 have little or no reduction in pH because no CO2 remains in solution at that pH.
Regards
Ashtree
"Any water can be made potable if you filter it through enough money"