mechengstudent99
Student
- Aug 29, 2021
- 3
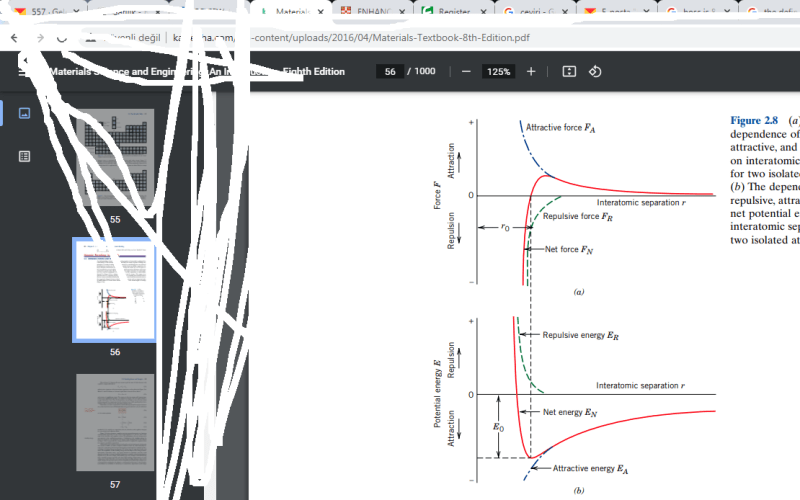
Hi guys i know its a very basic concept but i didnt quite understand this graph.
The graph shows us to two isolated atoms and their forces applies on them according to the distance between them.
My confussion is when the distance beetween two atoms is R0 there is no net force applied on them but how could still be a potential energy I thought that the potentail energy should be zero when the distance is R0 cause atoms dont want to move so they dont want to do work .(its obviosly wrong
