takiyasamsama
Chemical
Hi,
I have received info from client and we are required to find the enthalpy for the mixed flow (2 phase flow; liquid and vapor). The difficult thing for me is to find the total enthalpy for the mixed flow in kJ/kg. Client has given the heat curve in two separate phase (liquid and vapor) and enthalpy in kJ/kgmole. So now I'm having problem to find the enthalpy in kJ/kgmole. How do I convert this into combined enthalpy in kJ/kgmole.
I would really appreciate if anyone here could help me figure out this one. Attached image is what I'm referring to.
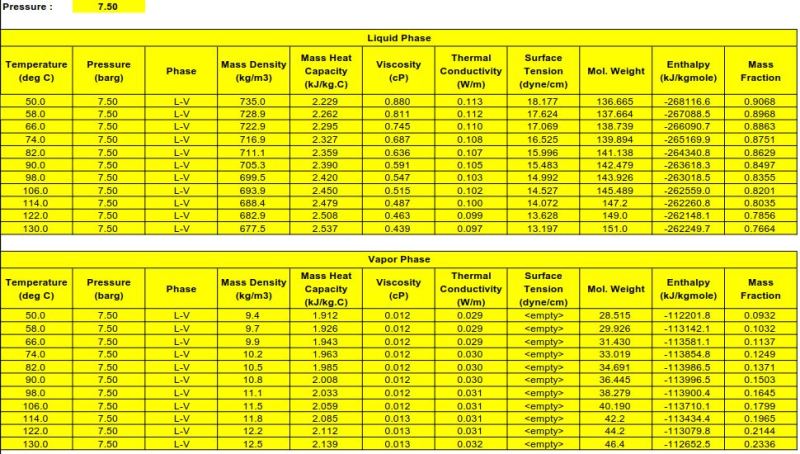
I have received info from client and we are required to find the enthalpy for the mixed flow (2 phase flow; liquid and vapor). The difficult thing for me is to find the total enthalpy for the mixed flow in kJ/kg. Client has given the heat curve in two separate phase (liquid and vapor) and enthalpy in kJ/kgmole. So now I'm having problem to find the enthalpy in kJ/kgmole. How do I convert this into combined enthalpy in kJ/kgmole.
I would really appreciate if anyone here could help me figure out this one. Attached image is what I'm referring to.

